STACLEAR
The First FDA-Cleared Syringe For Intravitreal Use
Designed For:
Ophthalmologists
Compounding Pharmacies
Ophthalmic Drug Companies

WHY INVENT AN INTRAVITREAL SYRINGE?
In 2018, the Food & Drug Administration (FDA) issued new, more stringent guidelines around compounding drugs for intraocular injections, adding regulatory risk.
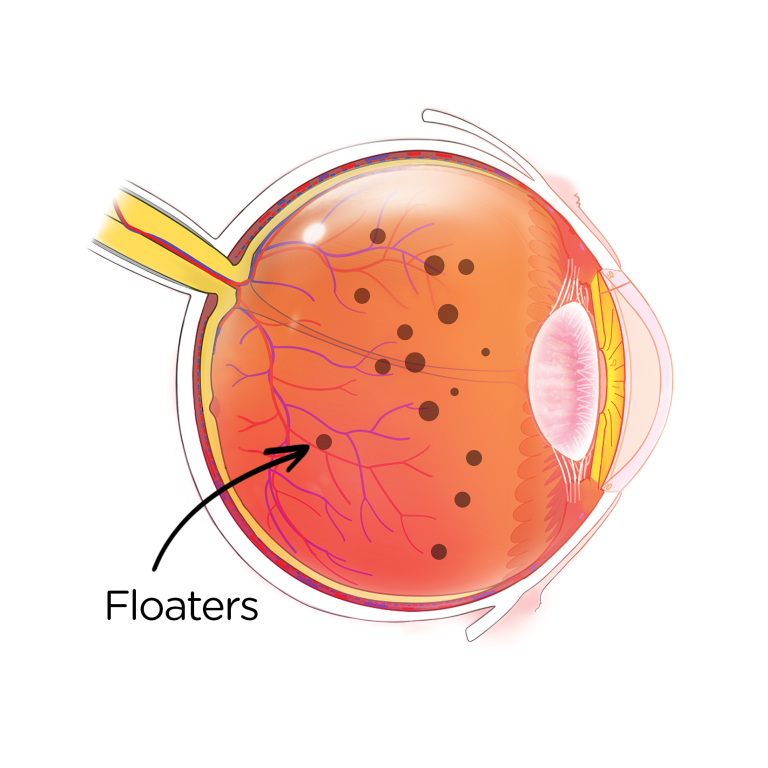
Floaters In The Eye Reported
In late 2016, the American Society of Retina Specialist (ASRS) alerted its members to the risk of floaters and intraocular pressure caused by liquid silicone oil -- the lubricant in generic insulin syringes.
The FDA Takes Steps To Ensure Patient Safety
Insurance companies started requiring that retina specialists have their patients sign a liability waiver before the procedure in view of this new liability risk.
The Industry Responded

StaClear meets the Food & Drug Administration's strict particulate standards for intraocular medical devices.
Why Stop at One Problem, When We Can Fix Them All?
Our team worked closely with ophthalmologists and compounding pharmacies to understand ALL their requirements:
Smoother plunger movement
High accuracy large markings
Reduced dead space
Optimized packaging for customer needs
Comply with FDA Guidance for 503B Facilities
StaClear is Currently Available in:
Luer Slip (SC250LS)
Luer Lock (SC250LL) and
Attached Needle (SC250AN) Syringes

Luer Slip (SC250LS)
Luer Lock (SC250LL)
Now with new 0.025mL Graduation Mark!
Attached Needle (SC250AN)

StaClear: Superior to General Use Syringes
100x FEWER PARTICLES THAN GENERIC SYRINGES - Coating is anchored to the walls of the syringe versus liquid silicone oil in generic syringes.
CONTROLLED INJECTION EXPERIENCE - Elastomer piston ensures sealing integrity -- a risk in silicone-free syringes.
LARGE GRADUATION MARKS - This enables precision dosing (0.25ml syringe with 0.01ml graduations).
IMPROVED ACCURACY - vs. larger 1ml syringe types.
A LOW DEAD-SPACE SYRINGE DESIGN - Compare StaClear with syringes that require $10-15 of drug wastage due to dead space.
OPHTHALMIC-SAFE MATERIALS - Meets chemical and biocompatibility standards for ophthalmic use.

The StaClear Team
-

“After years of R&D and a rigorous FDA process, it is gratifying to see significant market interest for StaClear, and receive positive client feedback.”
VINAY SAKHRANI - Founder & CEO
-

"We have helped many medical device & pharma companies solve difficult lubricant challenges, but the StaClear product is my absolute favorite."
JACKSON THORNTON - Co-Founder & Director of Technology
-
"One of my mentors receives an intravitreal injection once a month for wet AMD. Bringing this syringe to market is personally important to me."
NEESHA MIRCHANDANI- Director of Communications
-

“Patients first. Then use Quality as a tool to meet physician expectations. That's what makes us different from every other ophthalmic drug delivery device."
PAUL J STOKES - Quality Manager
Have Questions About StaClear? Get in Touch.
I'm Vinay Sakhrani, CEO of StaClear,
Please let me know if you have any questions about StaClear. Our team usually responds within 1 business day.
This Research Was Funded by National Eye Institute.
The National Eye Institute (through its SBIR grant program) championed and funded this important research. Thanks to its support, StaClear is the first syringe designed for ophthalmologists and in full compliance with FDA Guidance for intraocular safety.